Cephasonics Engineering & Design Consulting Services
These services are specifically designed to help our partners:
1) Accelerate development and implementation of their concepts and ideas for ultrasound applications, and 2) Design and build specific customized versions of the Cephasonics system to meet their particular product design parameters. |
Cephasonics provides a wide range of highly-focused engineering design and development services focused on helping customers and partners accelerate the development and production of custom ultrasound solutions based on the Cephasonics ultrasound HW & SW platform.
These services provide partners and customers access to senior engineering team members at Cephasonics representing some of the finest minds in the industry related to ultrasound hardware, software, deep learning and algorithm development along with a comprehensive understanding of the capabilities of the Cephasonics hardware and software platforms.
We can provide short term assistance to complete structured engineering programs to assist in the design and development of new ultrasound products and applications from prototype to design for volume manufacturing. Engineering services can be billed on either an hourly project basis.
These services provide partners and customers access to senior engineering team members at Cephasonics representing some of the finest minds in the industry related to ultrasound hardware, software, deep learning and algorithm development along with a comprehensive understanding of the capabilities of the Cephasonics hardware and software platforms.
We can provide short term assistance to complete structured engineering programs to assist in the design and development of new ultrasound products and applications from prototype to design for volume manufacturing. Engineering services can be billed on either an hourly project basis.
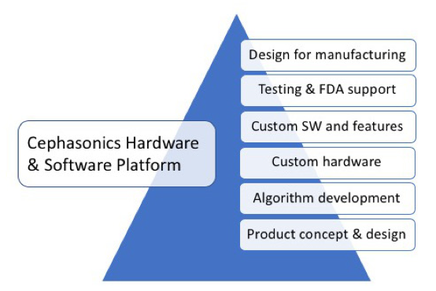
Custom hardware Ultrasound Concept Development and Design Feasibility
Joint design sessions to determine design concepts and constraints. Support for defining key design objectives and parameters as well as fundamental development requirements and approach. Analysis of overall approach and feasibility.
Algorithm Design and Development
Algorithms are essential to the success of any new ultrasound system/application. Cephasonics employs world recognized experts in ultrasound algorithm development. We provide special consulting and design assistance in developing and implementing new algorithms for displaying and analyzing ultrasound images.
Software Development
We provide custom ultrasound software development to provide you unique new features and customized APIs within the Cephasonics software environment to facilitate and support your end-user applications. We also provide extensive Python and Tensor-flow development to add AI and deep-learning to your applications.
Custom Hardware Design and Development
Packaging, design and prototype development of custom ultrasound systems based on the Cephasonics platform. Custom design of application specific HW features for the Cephasonics platform, i.e. motor controllers, integrated computers, displays and probes (would include partnership with key custom probe builders). Integration of specialized devices and components into the Cephasonics platform.
Testing and FDA Submission Support
Assistance in developing appropriate technical material for field testing processes as well as technical support for FDA submissions. Cephasonics-based ultrasound products have received FDA approval and we can assist you in submitting appropriate technical support materials for your FDA approval
Designs for Manufacturing
Develop BOMs for manufacturing. Create assembly instructions. We provide specialized assistance to turn Cephasonics-based prototypes and custom designs into manufacturer ready products. Whether it is working with manufacturers on bill of materials and component sourcing to custom product design, we can provide assistance to help overcome key challenges of moving from R&D and prototypes to full commercial production. Once your products are in full commercial production, Cephasonics can also provide continued backup technical assistance to your support staff for your installed customer systems.
Do you have specialized hardware needs for your product? We can provide design and production services for a wide variety of add-on hardware and software capabilities to the core Cephasonics platform including computer system integration, specialized controller boards, customer probes and more.
Joint design sessions to determine design concepts and constraints. Support for defining key design objectives and parameters as well as fundamental development requirements and approach. Analysis of overall approach and feasibility.
Algorithm Design and Development
Algorithms are essential to the success of any new ultrasound system/application. Cephasonics employs world recognized experts in ultrasound algorithm development. We provide special consulting and design assistance in developing and implementing new algorithms for displaying and analyzing ultrasound images.
Software Development
We provide custom ultrasound software development to provide you unique new features and customized APIs within the Cephasonics software environment to facilitate and support your end-user applications. We also provide extensive Python and Tensor-flow development to add AI and deep-learning to your applications.
Custom Hardware Design and Development
Packaging, design and prototype development of custom ultrasound systems based on the Cephasonics platform. Custom design of application specific HW features for the Cephasonics platform, i.e. motor controllers, integrated computers, displays and probes (would include partnership with key custom probe builders). Integration of specialized devices and components into the Cephasonics platform.
Testing and FDA Submission Support
Assistance in developing appropriate technical material for field testing processes as well as technical support for FDA submissions. Cephasonics-based ultrasound products have received FDA approval and we can assist you in submitting appropriate technical support materials for your FDA approval
Designs for Manufacturing
Develop BOMs for manufacturing. Create assembly instructions. We provide specialized assistance to turn Cephasonics-based prototypes and custom designs into manufacturer ready products. Whether it is working with manufacturers on bill of materials and component sourcing to custom product design, we can provide assistance to help overcome key challenges of moving from R&D and prototypes to full commercial production. Once your products are in full commercial production, Cephasonics can also provide continued backup technical assistance to your support staff for your installed customer systems.
Do you have specialized hardware needs for your product? We can provide design and production services for a wide variety of add-on hardware and software capabilities to the core Cephasonics platform including computer system integration, specialized controller boards, customer probes and more.

Cephasonics engineering and design group is headed by Richard Tobias, a worldwide recognized expert in ultrasound algorithms and system design.
He heads a team of hardware and software consultants who can help you design, develop and produce world-class ultrasound products.
The team brings wide expertise in ultrasound semiconductor design, hardware design, AI and deep learning, algorithms as well as a long history of bringing new products to market.
He heads a team of hardware and software consultants who can help you design, develop and produce world-class ultrasound products.
The team brings wide expertise in ultrasound semiconductor design, hardware design, AI and deep learning, algorithms as well as a long history of bringing new products to market.


